EP204 Introduction to Material and Energy Balances Quiz
Question 1
85 mol/s of liquid acetone (C3H6O) is entering a furnace at 40°C. It is burned completely with 25 % excess air flowing into the furnace at the same temperature. The burning process produces carbon dioxide (CO2) gas and water vapour leaving the furnace at 200°C. Compute the heat (kJ/s) that must be supplied to or removed from the furnace for the combustion process.
(25 marks)
Question 2
A liquid mixture containing 68 wt % cyclopentane (MW=70.13) and 32 wt % n-decane (MW=142.28) is fed to a continuous distillation column. The top product contains 98 wt % cyclopentane, while the bottom product consists of 3 wt % of the cyclopentane fed to the column. The volumetric flow rate of the feed stream is 1800 L/h and the specific gravity (SG) of the feed mixture is 0.853.
(a) Sketch a completely labelled process flow diagram for the distillation process.
(5 marks)
(b) Compute the mass flow rate (kg/h) of the overhead product stream and mole composition (mole fraction) of the bottom product stream.
(10 marks)
Question 3
In a mango juice manufacturing plant, the concentrated juice of 48 wt% solids is to be produced. An evaporator is used to concentrate the fresh juice with the composition 0.4 kg solids/kg water. A by-pass stream is introduced to the operation in order to maintain the desired solid concentration of the final mango juice. The output stream leaving the evaporator contains 60 wt% solids. Figure 1 illustrates the simplified process flow diagram of the process for the processing of 250 kg fresh mango juice.
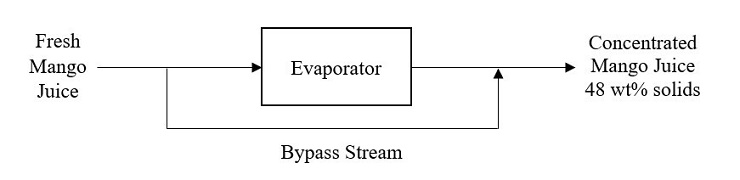
Figure 1
(a) Sketch a completely labelled process flowchart for the process.
(10 marks)
(b) Perform the degree of freedom analysis to all possible boundaries for the process.
(12 marks)
(c) Compute the mass of the juice that bypassed the evaporator and the mass of output stream leaving the evaporator.
(8 marks)
Question 4
The reaction of ammonia (NH3) with oxyegn is represented by the following overall reaction:
𝑁𝐻3+𝑂2 →𝐻𝑁𝑂3+𝐻2𝑂
The feed gas contains 30 mol NH3 (MW=17.031) and 55 mol pure oxygen (MW=16).
a) Determine the limiting reactant and calculate the percentage by which the other reactant is in excess.
(7 marks)
b) If 1.5 kg of HNO3 (MW=63.01) is formed, compute the percentage conversion (%) of the limiting reagent and the extent of reaction (mol) of the process.
10.8 kg/s of n-hexane (MW=86.17) vapour is flowing at 40°C into an adiabatic heat exchanger. A stream of superheated steam at a pressure of 10 bar and 330°C is used to heat up the n-hexane vapour to 270°C. The steam leaves the heat exchanger as saturated water at the same pressure.
1) Construct an inlet-outlet enthalpy table for the heating process.
(8 marks)
2) Compute the heat (kW) that must be transferred to the n-hexane to heat it from 40°C to 330°C.
(3 marks)
3) Assuming that all the energy transferred from the steam is used to heat the n-hexane, compute the rate of steam supply (kg/s) to the heat exchanger.
(4 marks) END OF QUESTION PAPER
